"Hi Jamie, March 20, 1997
I've been slow getting at this as, as you know, I was pretty discouraged at the loss of some of my papers. ... To Summarize 10 years of work and all I put into it and all I learned is somewhat sad. However, I have decided to view it as a short course in synaptic morphology, rather than a good chunk of my heart and soul. That makes it do-able.
Introduction
In 1970 (at age 37...) I had the temerity to answer an ad for Part Time-wanted biologically oriented and talented person. I was hired to do "frozen" sections. That is, to slice up and stain sections of rat brain that were essential for someone's experiment.
Did a lot of it, got very good at it,and in the doing, absorbed a great deal of neuroanatomy and neuroscience issues and information, as my desk was in the middle of what was then a tiny lab with graduate students and professor all crammed into a tiny space, so there was a lot of interaction and discussion.
Had just about sliced as many brains as I could handle (no longer a challenge) when I was asked if I'd like to do electron microscopy. Said of course,yes. By then was doing a fair amount of reading on my own.
Along here we moved to a much bigger lab, with many rooms for specialties. The woman who had been learning the lab aspects of e.m.(electron microscopy) was leaving and she taught me what she knew before she left. I found it very interesting and a challenge, particularly as I had never considered myself one who could do very fine, delicate hand work!! Turns out I could!
As I became capable of producing good material, the boss decided to do an experiment to decipher some clumps of material he had noted in neurons when he was using the microscope. At that point, he did perfusions, with me assisting, and he blocked out the portions of the brain he observed. Then I did the tissue prep, sectioning, staining. He did the work on the microscope and I did the film processing.
Then we had pictures to look at and study. The experiment was to study post mortum effects on the occurrence of these clumps. So perfusions were done immediately after death, 5 min after, 10 minutes after, etc. To see the effects upon the neurons as the brain continued to sort of disintegrate after death. Many labs at the time believed that it was adequate to "immerse" brain tissue in a fixative, rather than perfuse the animal with fixative, so this condition was included. (IT was very inadequate!)
It was a very instructive experiment and set the course for my attitude about the dangers of drawing absolute conclusions about synaptic morphology from what is always, no matter how well prepared, DEAD tissue!!. From then on, I was concerned about the possibility that certain aspects of fine structure seen by electron microscopy might be artifacts of the fixation procedure, or simply the result of the tissue being in a state of decomposition, no matter how slight.
I was to count incidences of these clumps of material in various conditions. In observing the photos, I started noting some other changes as a function of the particular condition. I noted a change in the postsynaptic density as post mortum time increased and a change in the curvature of the synapses. Told the boss and he decided to include these observations in the paper. I made a see-through gauge to measure synaptic curvature, did a lot of measuring of post synaptic densities and so on. I was included in the authorship and that was our first publication, Routtenberg and Tarrant.
At that time we were concentrating on the Caudate Nucleus, which I have referred to as "my first love". (My seemingly irreverent sense of humor has gotten me into trouble more than once!!!) We later moved on to the hippocampus as the main target of study.
(An aside - all the while that I was focussing on a tree (ie, a synapse), I always had a part of my brain working on the forest. In other words, while concentrating my efforts on the synapse, I always had the need to integrate the information into the whole, the way all the brain parts work together to produce environmentally useful behavior. Of particular interest to me was the behavior, or more properly the inhibition of behavior, that allowed us to think or act. Where in the brain could this uniquely (???) human behavior originate? At one point my candidate for this integration was the caudate nucleus. As you know, my interest in this issue, along with other relevant topics, resulted in "(Mitochondrial) Eve".)
I got more and more involved in the neuroscience literature, the questions and the work. I had found a world where I could use all my resources. The task was enormous, the opportunity and necessity for learning unending, yet the task was solvable. After all, we do see, think, talk and walk and the brain does it! Therefore figuring out how it does it must be answerable. And good data were reliable clues.
I read everything I could and more. Went to talks, sat in on courses, etc. Learned to use the microscope and lastly, started doing the perfusions (the one thing I really hated). But they had to be done and done well. To that end I devised an anesthesia mask for rats, to deliver O2 prior to perfusion. Eventually I was doing the whole works, including experiment design, paper writing, abstract writing, gave a talk in Toronto, and so on. The boss always did the statistics. (The only time he ventured back into the e.m. lab came when the inclusion of unanesthetized material in the study was deemed necessary. After doing a couple of the perfusions, I could not do any more so the boss did the rest.)
Having pretty significant published research projects, teaching e.m. techniques to others, etc, without having finished my undergraduate degree became a condition needing to be remedied. (I had been a physics major in the 50's, left college in my Junior year due to major family complications, worked for a bit, rode horses, and got married like all good 50's girls were supposed to do.)
I found a place, Lake Forest College, that would give returning students a financial break, and would take two years of credit from my earlier days at Goucher, and went back to school. Also continued lab work.
After fits and starts, got my degree in Psych (experimental) and then was back at the lab full time as a graduate student (1978). I had a very influential professor at Lake Forest who introduced me to the Gibsonian perspective of our interaction with the environment. She also encouraged me to continue with graduate study at the Univ. of Connecticut. My personal approach to many questions had always included our evolutionary past and I was able to apply this evolutionary perspective to many of the issues in brain, mind, language, and so on, at Lake Forest and at the lab.
This intro would not be complete without adding that I was totally captivated by the field, the questions, the interwoven data from neuroscience and psych. I had read and studied voraciously, in the lab and in school, absorbed and digested everything I read, and loved every minute of it. (except the perfusions!)
In addition, I really enjoyed the lab work, the almost daily fine work with my hands was something that I became so used to that when it was not available, I really suffered withdrawal. And to sit at the electron microscope and cruise through the brain, getting to know cell types almost as old friends, looking at the structures that enable us to see, hear, talk, think etc was an incredible trip. And I got immediate feedback as to the quality of my perfusions and tissue preparation, which was an excellent motivator to continually improve. There was nothing more frustrating than weeks of preparation and then getting to the scope and find that somewhere along the line you had messed up!
While I never was an expert in the mechanisms of the microscope, I think I was pretty expert at detecting and recording information viewed in the scope. (Two very knowledgeable women, Paloma and Donna, ran the two microscope facilities I used and they were the experts on the scopes themselves.) Scanning regions and detecting anomalies was something that produced the spinule work. My quite good spatial abilities enabled me to consistently identify regions and to find my way around serial sections quite easily. And it was a pretty good trick to be able to get the relevant serial sections to the scope to begin with!
That the information I gathered might be useful in solutions to the questions of "How does the brain work", was certainly a piece of icing on the cake.
========
The Research
So now for some synaptic details:
The standard view of the synapse, the one taught everywhere, was thus at the time I started all this:
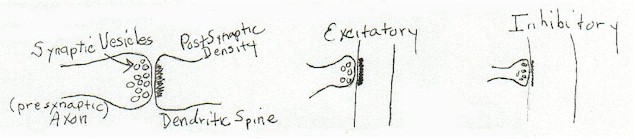 |
[diagram 1]
It was taught as fact that 'excitatory synapses' were synapses with round presynaptic vesicles and a thicker postsynaptic density than 'inhibitory synapses' which were considered to have smaller ovoid vesicles. Activity was assumed to move down the axon to the synapse and to traverse the synapse and be carried on passively by the post synaptic component to cell body. It was generally assumed that the suggestion of Hebb was correct. That strengthening these connections was the basis for learning and memory.
The first experiment we did showed me that the thickness of the post-synaptic density, as well as the shape of the synapse (and also the congregation of vesicles) was not a fixed immutable sign of anything. That many conditions could affect these "standard" parameters.
Through many hours of looking at micrographs and viewing directly in the microscope, I started noticing a synaptic structure that was not in the literature. It was present in certain synapses, those that terminated on dendritic spines, in both caudate nucleus and hippocampus. It always occurred in the midst of what was then considered to be the active zone of the synapse and always occurred in a break in the continuity of the post- synaptic density. It was first noted in the caudate nucleus on the spines on medium spiny cells, studied there and in the hippocampal dentate gyrus and also observed in other brain regions.
I started called the boss's attention to this, without much success, until I photographed a very clear structure that terminated in a "coated vesicle' on the axonal portion of the synaptic membrane. As coated vesicles had been implicated in the transport of large molecules across the membrane, the boss started paying attention again.
As I became able to spend more and more time looking for this entity, I began to notice other consistent attributes. It only occurred in synapses that had spine apparatus in the dendritic spine. The spine apparatus was a stack of membrane, almost always not seen in contact with the cell membrane. The function was unknown. The spinule also was not present in the hilus of the hippocampus, where the organization and cell type was entirely different than the molecular layer.
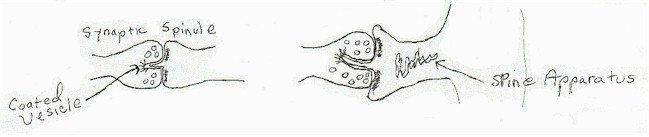 |
[diagram 2]
I began the process of determining the incidence of the "synaptic spinule" and characterizing it's appearance. This resulted in the first Tarrant and Routtenberg paper on the spinule and the talk at that Toronto Neuroscience Convention.
My healthy skepticism about the in vivo reality of synapse structure continued unabated all the while I was studying it! I considered every possibility that I could think of that the spinule was an artifact of something. Fixation, state of the animal at perfusion time, etc. I did many many perfusions, trying different parameters, counted the incidence in poorly fixed tissue as opposed to our best perfusions and so on. Searched all the literature, finally the publication by von Harreveld of a picture of a spinule in tissue prepared without chemical fixatives allowed me to finally conclude that it was, in all likelihood, not a fixation artifact.
Of course, all the while this was going on was (were) the question(s), if indeed it is there in the in vivo synapse, "What is it?? What is it's function?? Why the regular appearance?? - one in ten spine synapses in the hippo- campus dentate gyrus contained spinules. Why the association with the spine apparatus?? How could it appear in the midst of the post synaptic density?? Could we affect the incidence by experimental conditions?? What was the coated vesicle transporting?? And most puzzling, Where does the membrane come from, in the midst of the synapse, with no synaptic density?? Could it be supplied by the spine apparatus??"
The notion of a living synapse that was not a fixed permanent structure had become more certain to me. I had noted fine filaments that at times stretched from the spine apparatus into the post synaptic density, and into the spinule itself, and into the bulging spine cytoplasm that surrounded elaborate spinules. These fine filaments looked suspiciously like a filamentous aggregation of actin-like molecules.
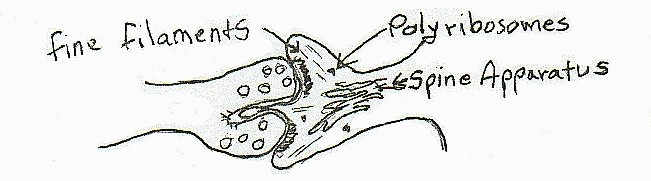 |
[diagram 3]
I say 'elaborate' because the spinules varied enormously in size and complexity. It was clear early on that the best way to study the spine was through serial sections and I began to routinely do serial sectioning for the study. As I did more and more I started worrying over another complication. The variation in size made the counting of the incidence in single sections a problem to my mind. I started detailing the geometry of slicing a section at various angles with the probability of coming across a spinule in a synapse with various size and shaped spinules. At this time I was also counting different sizes and shapes of spines, correlating spinule occurrence with spine head size and other possible consistencies in an effort to further characterize the spinule in order to provide more clues to its function.
 |
[diagram 4]
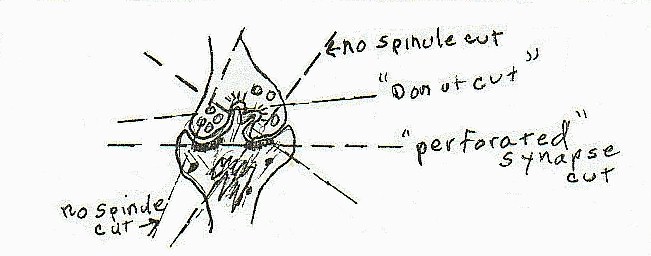
Again I concluded that because of this complication, the only way to study and count the spinules was with serial sections. Although without a precise determination of the thickness of a section (other than by color was the norm) there was no way to get precise measure of spinules per cubic measurement of tissue.
When it was necessary to study single sections, I finally determined that, as an entire spinule was generally not visible in this type of sectioning, that a reasonable way to count was to label the entities counted as "signs of synaptic spinule". These signs varied from a break in the interior of the post synaptic density to a doughnut shaped structure in the presynaptic terminal. Serial sections had shown these to portions of entire spinules of varying size, from the early sign which would be the break in the post synaptic density with a slight evagination of post synaptic membrane. This early phase, or a small slice of a more extensive spinule seen in another dimension, is apparently what is now called "perforated synapses".
 |
[diagram 5]
During these years, a constant monitoring of the literature produced additional clues as to the possible function of the spinule. Much data was accumulating about changes in synaptic numbers. Eye sutures, enriched environments, other forms of deprivation, of activity, all were demonstrating the lack of permanence in spines and therefore of the synapses upon them. They increased in number and decreased in number. All adding to the notion that the synapse, and in particular those with spinules, were mobile, active structures. That what we were viewing when we caught sight of a spinule was not a fixed state, but a slice in time of a process of change.
I had suggested various methods to see if we could manipulate the incidences of spinules. An enriched/ impoverished environment paradigm was one I had suggested for a while as there was evidence in the literature that this affected synapse number. While many of the studies of this type were in cortex, it seemed likely to me that hpc would be affected differentially also, particularly if the enriched environment contained a great variety of activity.
I also considered the possibility of distinguishing some aspects of plasticity and regional brain function by having conditions such as rats limited to a "mentally" impoverished environment, i.e. they would have extensive exercise on a treadmill but no other activity, versus a very complex enriched environment that offered exercise, puzzles, an enriched sensory environment that was relevant to rats, mazes, etc. I did not get the go ahead from Routtenberg on any of these suggestions.
I did get the go ahead on the following:
I decided to see what would happen to the spinule if I performed an axotomy on the dentate cells where most of my counting and observations took place. An electrolytic lesion I performed on the mossy fibers - although very minute in degree - created so much damage to the cells at a distance that I concluded that it was impossible to reach any interesting conclusions. (Although, there was an interesting observation that the destruction appeared sort of layered. In that there would be a long stretch of cells with extensive signs of destruction from the lesion, and then suddenly the destruction would cease and the next group of dentate cells would appear to be unaffected, as if there were either some sort of cellular barrier, or that the affected cells were connected in a way that the current was more invasive.)
I decided to try a hopefully less destructive technique and fashioned a tiny knife out of an electrode. I proceeded to stereotaxically slice through the mossy fiber output of the dentate granule cell, performing and axotomy. The question was, what would be the affect of this injury to other portions of the granule cell, of course, including the incidence of spinules on the cell's dendritic spines. (My boss called this 'very elegant' - a rare verbal compliment!)
Perfusions were done as fast as I could, following axotomy, and at various times following the operation.
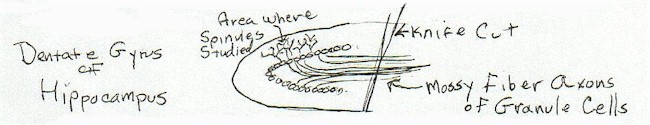 |
[diagram 6]
The results were interesting. The operation did affect the number of spinules as a function of time, following the operation. Changes were seen in even the first condition, termed '5 minutes', again indicating the mobility and lability of this structure.
I had been accumulating pictures of spine apparatus in various positions for quite a while; in the spine, at the base of the spine in the main dendritic shaft. I had quite a few pictures of the configuration in the dendritic spine that was rarely seen, never published that I know of. These were incidences of portions of the spine apparatus approaching and/or in contact with the post synaptic density. This unusual position of spine apparatus membrane, when observed, was often in a spine where there was a spinule in the synapse. More clues in the direction that the spine apparatus was the source of the extra membrane lacking post synaptic density that constituted the dendritic spine portion of the spinule. In the paper reporting this, Tarrant and Routtenberg, it was suggested that the spine apparatus and spinule were contributing to synaptic remodeling.
 |
[diagram 7]
Another area of interesting data was the growing realization of the importance of calcium in synaptic function. The analogy (homology) of the spine apparatus and the sarcoplasmic reticulum in muscle as internal storage and release sites of calcium had not escaped us. We were scooped in the suggestion by none other than Sir Francis Crick. This notion fit with the idea of the spinule as a mobile structure as well. The presence of calcium in the spine apparatus was later confirmed by calcium specific staining in another lab.
There had been growing interest in the phenomena of long term potentiation (LTP). This increase in the responsiveness of synapses was being suggested as a mechanism subserving long term memory. While the main stream view at the time was that anything significant at the synapse was a function of the presynaptic portion,more attention was being paid by some to the post-synaptic element as more than a passive recipient of neural activity. Which perhaps explains why the boss's interest at the beginning and perhaps throughout as far as I know, was mainly on the possibility of the retrograde transport of material from post synaptic to the presynaptic cell body, suggested perhaps by the coated vesicle usually seen on the invaginated presynaptic membrane.
More clues! It didn't seem unlikely (and hadn't for a while) that the synapses containing spinules were in some way more active than those without spinules. By now, other labs were investigating the incidence of spinules and some data from these labs could be considered. One study showed a greater incidence of synapses on dendritic shafts following LTP. Other studies were looking at spine shapes and incidence. Many of these were in the hippocampus and provided interesting data to add to speculation about the role of the spinule.
It was decided to get the hippocampal slice preparation up and running in the lab and to look at the incidence of spinules following stimulation that produced LTP. The work on the slice was done by another graduate student, and I was learning to do electrophysiological recording. It was a difficult task to get it up and running. I was a graduate student when we had material to look at. The experiment was eventually done by another lab with the boss's assistance ( I think) and indeed there was an increase in the incidence of synaptic spinules in the layer of hippocampus that had undergone stimulation producing LTP. (Hats thrown in the air time!!!!)
One other consideration had always been in my head, instigated by the original experiment we did, and further observations.
There seemed the possibility that the view we see in dead tissue of the post synaptic region of the spines is very far from the alive condition. That is, the increase in "clumping" seen as a function of post mortum time and the increase in the width of the post synaptic density indicated that perhaps, in the alive state, the components of the post synaptic density were a delicate structure that extended into the spine head, in contact perhaps with the spine apparatus. (Indicating) that this structure was the first to "clump" in the case of such a violent situation as 'death'.
While I was aware that the postsynaptic density exists, of course, in other than spine synapses, a post synaptic structure of varying complexity and size that extended into any post synaptic region might be the in vivo condition of all synapses. (!)
The possibility even existed that the post synaptic density was not so firmly attached to the post synaptic membrane as was assumed. This would have something to say to the question of the "appearance" of extra membrane in the midst of what was assumed to be a permanent attachment of post synaptic density to a specific region of membrane. The issues of membrane fluidity, receptor location, attachment and turnover, the contents of synaptosomal preparations, were all areas that I was studying with this thought in the corner of my mind. (I always liked to consider as many possibilities as seemed possible.)
My best guess however, other speculations not withstanding, of the role of the spinule in synaptic function had evolved over time as I did more experiments, read more, learned more and had available data from other labs.
My notion of a "slice in time" of a process remained viable as more information came in. In addition to the work that was specifically on the spinule, I had been making other observations all the time. The variation in size and shape of dendritic spines was a factor in forming my final best guess as to the role of spinules. Another important factor was the data from Lynch's lab with respect to the changing number of spine and shaft synapses following LTP stimulation.
An idea I had held in consideration for a long time (along with, "Is it an artifact??") was that it was a version of the dendritic "growth cone". That is, if the synapse, having reached it's maximum growth, it stimulated to the point that more growth is induced, what is the structure that develops? And how?
The metabolic considerations of maintaining synaptic function was always a consideration of mine. If indeed this was an active, changing region, it had to be supported metabolically with efficiency. This was a prime consideration. And was the basis for my best guess as to the reason there were only a certain number observed at one time. This was an important big job for the cell and would take much of it's mature metabolic resources. If indeed the spinule was a step in synaptic remodeling and dendritic spine synapse growth (and all that it implies) these were the necessary considerations.
So my final guess was the following process:
 |
"Dendritic spine synapse growth; Development of spine, synapse, and dendritic proliferation"
[diagram 8]
While at the time, there was no data to indicate that dendritic proliferation could occur as rapidly as was suggested by my notion, later data has shown that this rapid increase in dendritic areas does occur.
A recent brief synopsis of the effect of CFEB protein as signaling the delivery of postsynaptic components needed for more active synapses, complete with drawings of synapses, (which were pictured as those synapses with "indentations" !!) was quite gratifying. (Unsatisfyingly) it was however from an 'unreliable' source, the evening news! I hope to be able to get access to the paper or papers from which it came, if it did!
(Hoping to live long enough for the technology to develop that will enable the visualization of dendritic spine synaptic activity and growth in real time, in live tissue!!!)"
Sure this is more than you ever wanted to know, but I couldn't do it half-way! Sally (STC) Sally Tarrant Cobb
Index of MicroPhotographs (with margin comments) |
Hi Jamie -
Well this turned out to be kind-of fun. I started an addendum even, which is more personal stuff so will keep it on my computer --- I sure do miss the field. I must get to a library and see how my notion is holding up. Sally
