12 October 1992
The Royal Swedish Academy of Sciences has decided to
award the 1992 Nobel Prize in Chemistry
to Professor Rudolph A. Marcus, California Institute of Technology, Pasadena,
California, USA
for his contributions to the theory of electron transfer reactions in chemical systems
A THEORY CLOSE TO REALITY
Professor Rudolph A. Marcus is being rewarded for his theoretical work on
electron transfer - work which has greatly stimulated experimental developments in
chemistry. The processes Marcus has studied, the transfer of electrons between molecules
in solution, underlie a number of exceptionally important chemical phenomena, and the
practical consequences of his theory extend over all areas of chemistry. The Marcus theory
describes, and makes predictions concerning, such widely differing phenomena as the
fixation of light energy by green plants, photochemical production of fuel,
chemiluminescence ("cold light"), the conductivity of electrically conducting
polymers, corrosion, the methodology of electrochemical synthesis and analysis, and more.
From 1956 to 1965 Professor Marcus developed his theory for what is perhaps the simplest
chemical elementary process, the transfer of an electron between two molecules. No
chemical bonds are broken in such a reaction, but changes take place in the molecular
structure of the reacting molecules and their nearest neighbours. This molecular change
enables the electrons to jump between the molecules.
Professor Marcus found simple mathematical expressions for how the energy of the molecular
system is affected by these changes. With these he was able to calculate and explain the
greatly varying rates measured for electron transfer reactions. In the mathematical
connection the Marcus theory makes between theoretical and experimental quantities,
experimental chemists gained a valuable tool. The theory has proved useful in the
interpretation of many chemical phenomena, even though it was initially controversial at
some points. Certain predictions turned out to conflict with what the chemists had
expected, and were also difficult to confirm experimentally. We had to wait for the final
experimental confirmation until the latter part of the 1980s.
Background
When two molecules in a solution exchange one or more electrons, there is a
reduction/oxidation process (redox process) in which one molecule accepts the electrons
(reduction) and the other loses them (oxidation). Several different mechanisms can
underlie such reactions. The simplest is the transfer of one single electron from one
molecule to another. Changes take place in the structure, both in the reacting molecules
and in those of the solution medium. Because of all these changes the energy of the
molecular system rises temporarily and enables the electron to jump between the molecules.
Energy must thus be supplied for the electron to be able to cross an energy barrier. The
size of the energy barrier determines the speed of the reaction. An electron transfer of
this kind is the simplest chemical elementary process, and is eminently suitable for
theoretical studies.
At the beginning of the 1950s it was possible to determine the speed of a number of
electron transfers between inorganic ions. Some of the reactions turned out to be very
slow, which was surprising in view of the fact that only one electron changed places. It
was considered at the time that such an insignificant change should not give rise to any
large energy barrier.
The prizewinner's contributions
From 1956 to 1965 Marcus published a series of papers on electron transfer reactions. His
work led to the solution of the problem of greatly varying reaction rates.
Marcus made two assumptions about the reacting molecules. First, they had to be very
loosely bonded to each other during the course of the reaction for classical
physical-chemical theory to apply. Secondly, he assumed that it is the solvent molecules
in the immediate vicinity that change their positions, thus increasing the energy in the
molecular system. The electron can only jump between two states that have the same energy,
and this condition can be fulfilled only by increasing the energy for both molecules.
Marcus found a simple mathematical formula for calculating this energy change and was thus
also able to calculate the size of the energy barrier. Somewhat later he extended the
theory to include the energy associated with changes in the bonds of the reacting
molecules.
In addition, Marcus further developed his model by showing that energy barriers could be
calculated as a sum of two terms characterising each of the two components of the
reaction. Lastly, he derived a general connection between electron transfer speed and the
free energy change of the reaction, its "driving force".
The general equation is quadratic and describes a parabola (see figure). The formula has
the interesting consequence, unexpected by the chemist's intuition, that, for a
sufficiently large driving force, the reaction ought to take place more slowly the larger
the driving force becomes. This area even received a special name, "the inverted
region". In the 1960s this prediction ran completely counter to chemists'
expectations and, in addition, it was difficult to study reactions of this type
experimentally. Marcus himself proposed in 1965 that chemiluminescence reactions of a
certain type ought to represent the inverted region, but it was not until the end of the
1980s that other, more convincing, experimental verifications could be made.
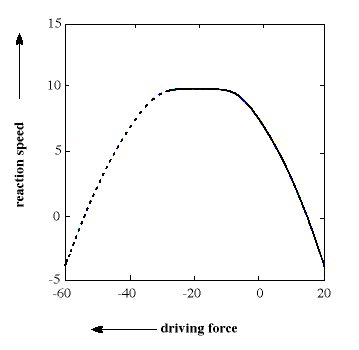
The figure shows a parabola which illustrates the connection between reaction speed
(on a logarithmic scale) and driving force (expressed in kcal/mol) for an electron
transfer reaction. In the left-hand part of the parabola (dashed line, the inverted
region) reaction rate decreases with increasing driving force, a prediction that chemists
long found difficult to accept and confirm.

